The Photocatalysis
The Photocatalysis is a natural phenomenon in which a substance, known as photocatalyst, modifies the speed of a chemical reaction through the action of light (natural or artificial). Most applications use photocatalysts with a dioxide titanium base (TiO2) which need UV light to activate the process.
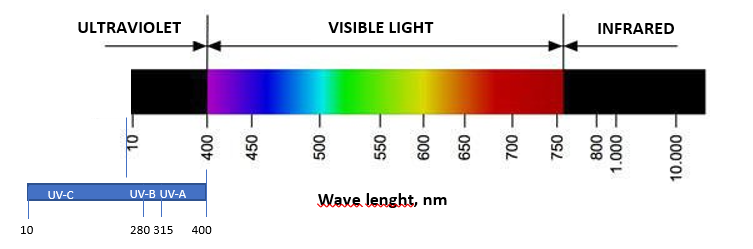
THE NEW PHOTOCATALYST ACTIVE IN THE VISIBLE LIGHT SPECTRUM – The development of a new photocatalyst tungsten trioxide based (WO3), significantly incremented the photocatalysis effectiveness and solved the problem of the UV light employment. When exposed to visible light, the WO3 absorbs and converts the light energy into electron and electron-holes. WO3 reacts with water (air humidity) to produce hydroxyl radicals (OH-) and with oxygen to produce superoxide anions (O2-). Billions of these high oxidant species are created in billionths of a second and work to decompose matter at molecular level. The result is an effective decomposition of the polluting organic and inorganic substances (comparable to all fine particles PM2.5-PM10), of microbes, viruses, nitrogen oxides, aromatic polycondensates, sulphur dioxide, carbon monoxide, formaldehyde, methanol, ethanol, benzene, ethylbenzene, nitrogen monoxide and dioxide, etc. The strong oxidative effect permits to use the tungsten trioxide based photocatalyst as photocatalytic disinfectant. Although several research studied the photocatalytic inactivation of bacteria, only a few tackle the virus inactivation. The effects resulted from the employment of photocatalytic solutions on viruses are reported below.
Photocatalysis and mycroorganisms
It has been proved that photocatalysis can trigger a degradation in case of simple compounds (protein and DNA), an inhibitory effect in case of viruses and bacteria, and an anti-cancer effect in the case of complex cells, like in regards of pollen and spores causing allergies.
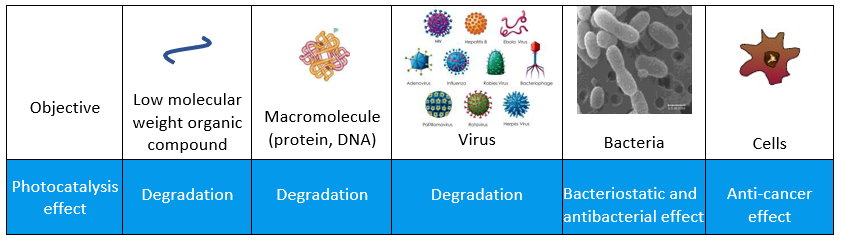
Reseach on virus transformation through photocatalysis has been executed in aqueous or liquid environment or with the method of direct contact between the organism and the surface. There are two levels of photocatalytic attack:
-
PHOTOINACTIVATION or PHOTODISACTIVATION, resulting in a DISINFECTING effect
-
DECOMPOSITION/KILLING viral cell, resulting in a STERILIZING effect
The inactivation mechanism of the virus via photocatalysis has still to be definitively cleared, although it has already been proved the effectiveness of the system with laboratory tests by the use of several microorganisms types and having achieved an almost complete result in the attack.
It seems like that the attack starts on the virus particles through their absorption on the catalyst surface, following the attack to the protein capsid and the virus binding sites (Redox direct attack). According to others, the inactivation behaviour of viruses is regulated by the hydroxylic radicals •O2 – and OH• and by Reactive Oxygen Species (ROS), such as •O2 -, OH– H2O •HO, free in the mass phase – and not by those bounded to the catalyst surface. The following decomposition mechanism involves the degradation of the cellular wall and of the cytoplasmic membrane, as a consequence of the ROS production. This process initially brings to the leakage of cell content, after to cell lysis, until a complete mineralization of the organism. The closer the contact between the virus and the catalyst, the more effective the killing will be.
Although taking into consideration the environmental conditions of the interface, the reactive species have a range that can reach 2 mm from the active surface.
The importance of the surface on which to install the photocatalyst
The photocatalytic surface exploited for the reaction is composed of a matrix or substrate containing uniformly dispersed photocatalyst particles, or else made of a thin film completing coating the substrate.
Not every material is adequate for this scope, depending on the chemical stability of the agents in contact with the surface or matrix in which their inserted. Those have chemical stability on plastics, fibre, fabrics and metals (with a nearly neutral pH).
The materials’ surface may be further modified to enhance the degrading effect.
For instance, it has been proved that the killing activity of the microorganisms can be further boosted in presence of other antimicrobial agents, such as silica and vitreous substances containing copper (Cu+ e Cu2+) and silver (Ag+) ions, or products composed of complexed metallic silver (colloidal silver), which behave as additional reservoir of active substances capable of attacking microorganisms.
Concerning air treatment, it is important that the filters have the widest contact surface with air and low resistance to airflow, for energetic consumption and noise reasons.